Experimental Report III on PTFE Membrane Deamination Test at High Temperature
Release time:
2024-07-23
When the amount of alkali added to the raw water increases, the ammonium root in the raw water will fully react with the hydroxide to generate ammonia, which increases the air permeability of the deammoniated membrane, resulting in a decrease in the ammonia nitrogen produced in the water. When the influent flow rate decreases, the air permeability per unit area of the deamination membrane increases, and the deamination effect is enhanced, which causes the decrease of ammonia nitrogen in water production.
1. Introduction to process technology
The deamination membrane product developed by Conano uses PTFE hollow fiber membrane with extremely high specific surface area as deamination interface. The ammonia removal membrane is equipped with a large number of hollow fibers, and there are tiny holes on the wall of the fiber. Water cannot pass through such small holes under the action of surface tension, while ammonia molecules can pass through.
Membrane absorption deamination technology is a new type of high-efficiency deamination technology that combines physical or chemical absorption and membrane separation. First of all, adding a certain amount of alkali solution to adjust the pH value of ammonia nitrogen wastewater, ammonia nitrogen in water there is a dissociation equilibrium, with the increase of pH, ammonia in water dissociation, when the pH is higher than 11, 98% of ammonia in wastewater dissociation into free NH3. After adjusting the pH value, ammonia nitrogen wastewater and sulfuric acid absorption liquid with ammonia dissociation enter the tube side and shell side of the membrane contactor respectively. As ammonia in the wastewater has been dissociated into NH3,NH3 will gradually volatilize from the gas-liquid interface and diffuse through the membrane pores of the hydrophobic porous PTFE membrane into dilute sulfuric acid to be absorbed.
The microscopic reaction process is shown in Figure 1.
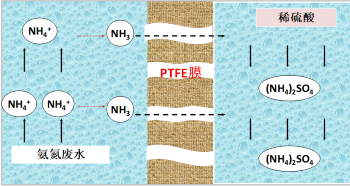
The hydrophobic PTFE membrane of hollow fiber structure has a very high specific surface area and provides a mass transfer interface for two-phase mass transfer. Because PTFE material has good hydrophobicity and chemical stability, compared with other membrane materials, such as PP or PVDF, PTFE membrane has a long service life and stable operation performance.
In the traditional blowing off process, the high ammonia nitrogen wastewater after adding alkali dissociation first enters the blowing tower, and a large amount of compressed air is used for blowing off. The exhaust gas after blowing off needs to enter the acid absorption tower to absorb ammonia in the exhaust gas, so as not to enter the atmospheric pollution environment.
In the membrane absorption process, the membrane contactor completely replaces the functions of the blowing tower and the desorption tower, and combines it into a membrane contactor as a whole. In the membrane contactor, ammonia desorption and absorption processes can be carried out at the same time. Because the PTFE hollow fiber membrane in the membrane contactor is a filament structure, the contact area is large, and the reaction efficiency is improved, which can greatly reduce the equipment footprint. In addition, since the membrane absorption process is not used to blow off air, it is not.
It will bring secondary pollution to the process and has a strong technical advantage. See Fig. 2 for process engineering.
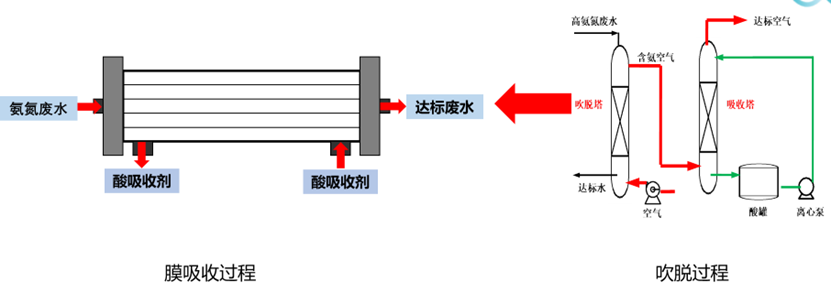
The main advantages of membrane absorption technology are:
The mass transfer driving force is large, and the ammonia nitrogen removal efficiency is high. The absorption of ammonia by the absorption liquid is a rapid reaction, and the concentration of free ammonia on the absorption liquid side is strictly zero, which provides the maximum driving force for the mass transfer process, so that the ammonia nitrogen in the wastewater can be effectively removed below the national allowable discharge standard or specified concentration.
In addition to sulfuric acid, hydrochloric acid, nitric acid, renewable absorbents, etc. can also be used as the absorption liquid. The corresponding by-products are ammonium chloride, ammonium nitrate, ammonia, etc., with high concentration and purity, which can be recycled or exported to reduce the cost of wastewater treatment.
Low energy consumption and low operating cost.
When the traditional stripping or stripping process needs to adjust the pH value of wastewater, generally can not use hydrated lime, can only use NaOH. This is because the solubility of Ca(OH)2 decreases with the increase of temperature in the stripping process, which makes it precipitate from the wastewater. In the stripping process, Ca(OH)2 in the wastewater reacts with CO2 in the air to generate insoluble CaCO3, which can cause fouling of tower internals and blockage of tower, affecting the service life and normal operation of the equipment. Even if anti-fouling tower internals are used, the tower equipment needs to be cleaned frequently. The stable membrane absorption process feed liquid does not need to contact with air, so there is no above problem, so the use of lime to adjust the pH value of the solution, so that the operating cost is greatly saved.
Ammonia in the wastewater into ammonium in the absorption solution only needs to pass through a layer of hydrophobic membrane with a thickness of 200 microns, and does not need to encourage a large amount of air to contact with the wastewater and carry out in a closed system. Therefore, there is no problem that toxic and odorant components in the wastewater are blown into the atmosphere, thus avoiding secondary pollution and having great environmental benefits.
The use of PTFE membrane, corrosion resistance, acid and alkali resistance, stable operation.
2. Purpose of the experiment
(1) The feasibility of wastewater treatment was verified by PTFE membrane deamination test;
(2) Make a relevant estimate of the overall operating costs.
3. Experimental drugs and equipment
Test raw material: ammonia nitrogen wastewater;
Test equipment: Conano PTFE membrane deamination test device;
Experimental aids: heating rod, digital thermometer, pH meter.
4. Experimental steps
The overall process route is as follows:
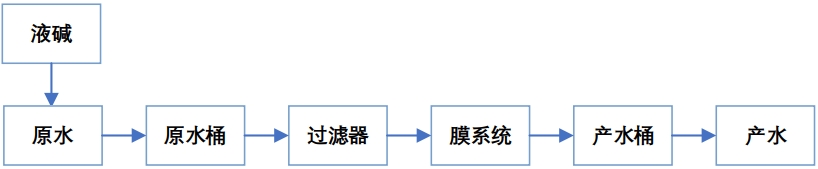
5. Record and analysis of membrane deamination experiment
5.1 experimental results (tube side)
(1) raw water at room temperature pH = 7.85, ammonia nitrogen content in 225.6ppm;
(2) Configure sulfuric acid absorption solution with 2000mL pH = 1, raise the raw water temperature to 70 ℃, and set the sulfuric acid absorption solution flow rate to about 42L/h
- When the influent 15.6 is set to L/h, the amount of alkali added is 1.5g/L(pH = 9.03), and the effluent ammonia nitrogen is 46.7mg/L;
- When the influent 12.0 is set to L/h, the amount of alkali added is 1.5g/L(pH = 9.03), and the effluent ammonia nitrogen is 21.0mg/L;
- When the influent 9.0 is set to L/h, the amount of alkali added is 1.5g/L(pH = 9.03), and the effluent ammonia nitrogen is 12.5mg/L;
- When the influent 15.6 is set to L/h, the amount of alkali added is 1.75g/L(pH = 9.12), and the effluent ammonia nitrogen is 22.9mg/L;
- When the influent 12.0 is set to L/h, the amount of alkali added is 1.75g/L(pH = 9.12), and the effluent ammonia nitrogen is 13.8mg/L;
- When the influent 9.0 is set to L/h, the amount of alkali added is 1.75g/L(pH = 9.12), and the effluent ammonia nitrogen is 10.1mg/L;
(3) With the operation of the device, the volume of sulfuric acid absorption liquid decreased significantly, from 1550mL to 1300mL.
|
Temperature = 70 ℃, raw water flow = 15.6L/h, acid circulation volume = 42 L/h, influent pH ≈ 9.03(1.5g/L) |
|||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 225.6 | / | / |
| Primary effluent | 46.7 | 79.30% | / |
| Temperature = 70 ℃, raw water flow rate = 12.0 L/h, acid circulation rate = 42L/h, influent pH ≈ 9.03(1.5g/L) | |||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 225.6 | / | / |
| Primary effluent | 21.0 | 90.69% | / |
| Temperature = 70 ℃, raw water flow = 9.0L/h, acid circulation volume = 42 L/h, influent pH ≈ 9.03(1.5g/L) | |||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 225.6 | / | / |
| Primary effluent | 12.5 | 94.46% | / |
| Temperature = 70 ℃, raw water flow = 15.6L/h, acid circulation volume = 42 L/h, influent pH ≈ 9.12(1.5g/L) | |||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 225.6 | / | / |
| Primary effluent | 22.9 | 89.85% | / |
| Temperature = 70 ℃, raw water flow = 12.0L/h, acid circulation volume = 42 L/h, influent pH ≈ 9.12(1.75g/L) | |||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 225.6 | / | / |
| Primary effluent | 13.8 | 93.88% | / |
| Temperature = 70 ℃, raw water flow = 15.6L/h, acid circulation volume = 42 L/h, influent pH ≈ 9.12(1.5g/L) | |||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 225.6 | / | / |
| Primary effluent | 10.1 | 95.52% | / |
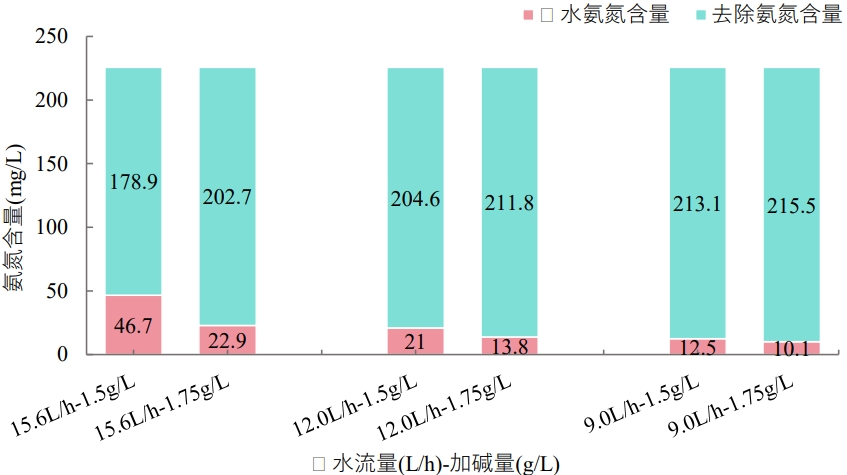
Fig. 1 Change of influent flow/alkali addition-ammonia nitrogen content
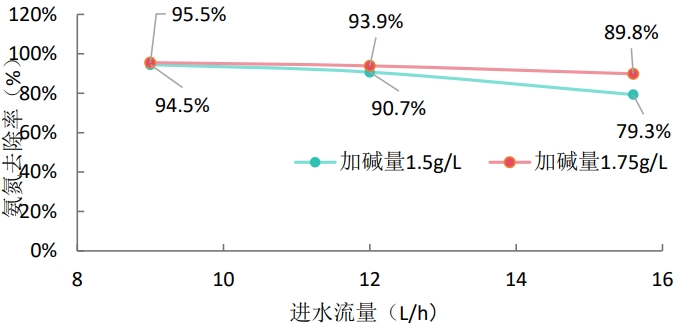
Fig.2 Flow rate of influent-ammonia nitrogen removal rate
5.2 experiment analysis and matters needing attention
(1) The ammonia nitrogen emission standard of the Project is 10ppm. In this experiment, under the conditions of raw water temperature 70 ℃, alkali addition 1.75g/L, and acid absorption solution pH = 1: when the raw water flow rate is 9.0L/h, the ammonia nitrogen in the effluent after stable operation of the primary membrane is 10mg/L;
(2) During the experiment, it was found that the volume of sulfuric acid absorption liquid gradually decreased, as shown in the following figure. The reason may be that the water temperature increased during the deamination process, while the salt concentration of raw water was high, and the steam in the sulfuric acid absorption liquid penetrated into the membrane and discharged with the produced water.
(3) During the experiment, it was found that after adding NaOH under heating conditions, the raw water became turbid and the residual solid was zinc carbonate.

6. Summary and plan
6.1. Experiment summary
(1) Under the conditions of raw water temperature 70 ℃ and acid absorption solution pH = 1, when the alkali addition amount of wastewater is about 1.75g/L(pH = 9.12) and the raw water flow rate is 9.0L/h, the ammonia nitrogen content of water produced by primary membrane deamination is 10mg/L after stable operation. 25L of raw water was treated, and about 0.25L of sulfuric acid absorbing solution was reduced.
(2) This small test has changed from the previous conditions. The main reason is that the ammonia nitrogen content of the raw water used this time (225.6ppm) has doubled from the previous raw water ammonia nitrogen (102.8ppm), so the alkali addition has increased by 0.25g/L. When the amount of alkali added is 1.75g/L, the water production can basically meet the drainage requirements.
(3) Analysis according to the results of the small test:
- When the amount of alkali added to the raw water increases, the ammonium root in the raw water will fully react with the hydroxide to generate ammonia, which increases the air permeability of the deammoniated membrane, resulting in a decrease in the ammonia nitrogen produced in the water.
- When the influent flow rate decreases, the air permeability per unit area of the deamination membrane increases, and the deamination effect is enhanced, which causes the decrease of ammonia nitrogen in water production.
6.2. Summary of experiment plan
It is planned that the next experiment will be to feed water into the shell layer. According to the same conditions of this experiment, compare the results of the tube process experiment.
Latest News












