Experimental Report on High Temperature PTFE Membrane Deamination Test
Release time:
2024-07-16
In this small-scale experiment, under the conditions of raw water temperature 70 ℃, alkali addition amount of wastewater about 2g/L(pH = 9.29), and acid absorption solution pH = 1.5: when the raw water flow rate is 16.8L/h, the ammonia nitrogen content of water produced by primary membrane deamination is 7.4mg/L after 10 minutes of stable operation, which meets the ammonia nitrogen requirement of drainage.
1. Introduction to process technology
The deamination membrane product developed by Conano uses PTFE hollow fiber membrane with extremely high specific surface area as deamination interface. The ammonia removal membrane is equipped with a large number of hollow fibers, and there are tiny holes on the wall of the fiber. Water cannot pass through such small holes under the action of surface tension, while ammonia molecules can pass through.
Membrane absorption deamination technology is a new type of high-efficiency deamination technology that combines physical or chemical absorption and membrane separation. First of all, adding a certain amount of alkali solution to adjust the pH value of ammonia nitrogen wastewater, ammonia nitrogen in water there is a dissociation equilibrium, with the increase of pH, ammonia in water dissociation, when the pH is higher than 11, 98% of ammonia in wastewater dissociation into free NH3. After adjusting the pH value, ammonia nitrogen wastewater and sulfuric acid absorption liquid with ammonia dissociation enter the tube side and shell side of the membrane contactor respectively. As ammonia in the wastewater has been dissociated into NH3,NH3 will gradually volatilize from the gas-liquid interface and diffuse through the membrane pores of the hydrophobic porous PTFE membrane into dilute sulfuric acid to be absorbed. The microscopic reaction process is shown in Figure 1.
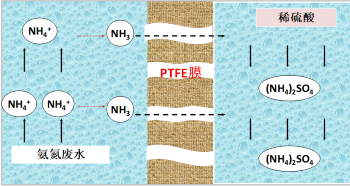
The hydrophobic PTFE membrane of hollow fiber structure has a very high specific surface area and provides a mass transfer interface for two-phase mass transfer. Because PTFE material has good hydrophobicity and chemical stability, compared with other membrane materials, such as PP or PVDF, PTFE membrane has a long service life and stable operation performance.
In the traditional blowing off process, the high ammonia nitrogen wastewater after adding alkali dissociation first enters the blowing tower, and a large amount of compressed air is used for blowing off. The exhaust gas after blowing off needs to enter the acid absorption tower to absorb ammonia in the exhaust gas, so as not to enter the atmospheric pollution environment.
In the membrane absorption process, the membrane contactor completely replaces the functions of the blowing tower and the desorption tower, and combines it into a membrane contactor as a whole. In the membrane contactor, ammonia desorption and absorption processes can be carried out at the same time. Because the PTFE hollow fiber membrane in the membrane contactor is a filament structure, the contact area is large, and the reaction efficiency is improved, which can greatly reduce the equipment footprint. In addition, since the membrane absorption process is not used to blow off air, it is not.
It will bring secondary pollution to the process and has a strong technical advantage. See Fig. 2 for process engineering.
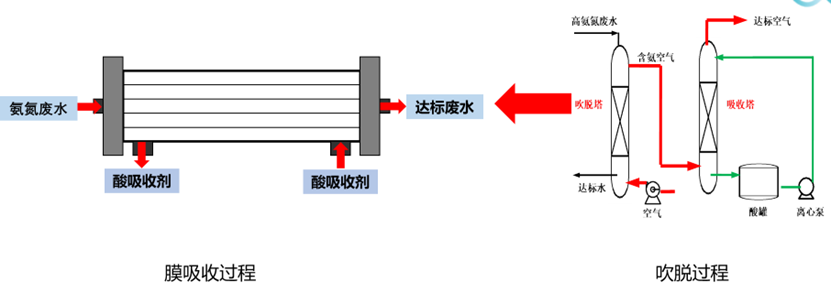
The main advantages of membrane absorption technology are:
The mass transfer driving force is large, and the ammonia nitrogen removal efficiency is high. The absorption of ammonia by the absorption liquid is a rapid reaction, and the concentration of free ammonia on the absorption liquid side is strictly zero, which provides the maximum driving force for the mass transfer process, so that the ammonia nitrogen in the wastewater can be effectively removed below the national allowable discharge standard or specified concentration.
In addition to sulfuric acid, hydrochloric acid, nitric acid, renewable absorbents, etc. can also be used as the absorption liquid. The corresponding by-products are ammonium chloride, ammonium nitrate, ammonia, etc., with high concentration and purity, which can be recycled or exported to reduce the cost of wastewater treatment.
Low energy consumption and low operating cost.
When the traditional stripping or stripping process needs to adjust the pH value of wastewater, generally can not use hydrated lime, can only use NaOH. This is because the solubility of Ca(OH)2 decreases with the increase of temperature in the stripping process, which makes it precipitate from the wastewater. In the stripping process, Ca(OH)2 in the wastewater reacts with CO2 in the air to generate insoluble CaCO3, which can cause fouling of tower internals and blockage of tower, affecting the service life and normal operation of the equipment. Even if anti-fouling tower internals are used, the tower equipment needs to be cleaned frequently. The stable membrane absorption process feed liquid does not need to contact with air, so there is no above problem, so the use of lime to adjust the pH value of the solution, so that the operating cost is greatly saved.
Ammonia in the wastewater into ammonium in the absorption solution only needs to pass through a layer of hydrophobic membrane with a thickness of 200 microns, and does not need to encourage a large amount of air to contact with the wastewater and carry out in a closed system. Therefore, there is no problem that toxic and odorant components in the wastewater are blown into the atmosphere, thus avoiding secondary pollution and having great environmental benefits.
The use of PTFE membrane, corrosion resistance, acid and alkali resistance, stable operation.
2. Purpose of the experiment
(1) The feasibility of wastewater treatment was verified by PTFE membrane deamination test;
(2) Make a relevant estimate of the overall operating costs.
3. Experimental drugs and equipment
Test raw material: ammonia nitrogen wastewater;
Test equipment: Conano PTFE membrane deamination test device;
Experimental aids: heating rod, digital thermometer, pH meter.
4. Experimental steps
The overall process route is as follows:
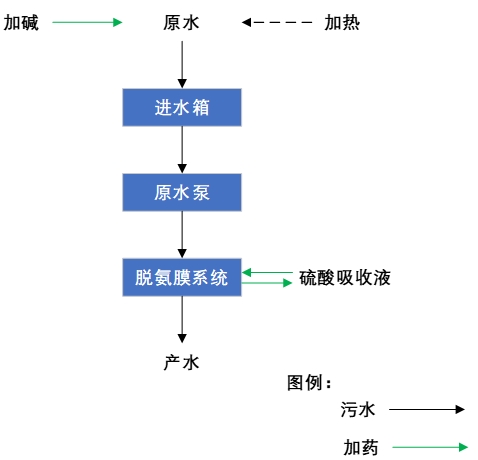
5. Record and analysis of membrane deamination experiment
5.1 experimental results (tube side)
(1) raw water at room temperature pH = 8.39, ammonia nitrogen content in 120ppm;
(2) If the pH of raw water is raised to 11.5 at room temperature, it is theoretically necessary to add 0.1265gNaOH to 1L of raw water, and the pH can reach 11.28 only after 7.5(gNaOH)/(L water) is actually added at 55 ℃;
(3) The pH of the raw water is 9.29 at room temperature, the sulfuric acid absorption solution 1250mL pH = 1.5 is configured, the temperature of the raw water is raised to 70 ℃, the alkali amount is 2g/L(pH = 9.29), the ammonia nitrogen content is 85.6ppm, and the flow rate of the sulfuric acid absorption solution is set to about 42L/h;
- When the influent 16.8 is set to L/h, the effluent ammonia nitrogen is 7.4mg/L;
- When the influent 13.8 is set to L/h, the effluent ammonia nitrogen is 2.9mg/L;
- When the inlet 12.0L/h is set, the effluent ammonia nitrogen is not detected;
(4) With the operation of the device, the volume of sulfuric acid absorption liquid is significantly reduced.
Table 1 Experimental data
| Temperature = 70 ℃, raw water flow rate = 16.8L/h, acid circulation rate = 42 L/h, influent pH ≈ 9.29 | |||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 85.6 | / | / |
| Primary effluent | 7.4 | 91.4% | / |
| Temperature = 70 ℃, raw water flow rate = 13.8 L/h, acid circulation rate = 42L/h, influent pH ≈ 9.29 | |||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 85.6 | / | / |
| Primary effluent | 2.9 | 96.6% | / |
| Temperature = 70 ℃, raw water flow rate = 12.0L/h, acid circulation rate = 42 L/h, influent pH ≈ 9.29 | |||
| / | Ammonia nitrogen (mg/L) | removal rate | Remarks |
| Raw water | 85.6 | / | / |
| Primary effluent | ND | 99.9% | / |
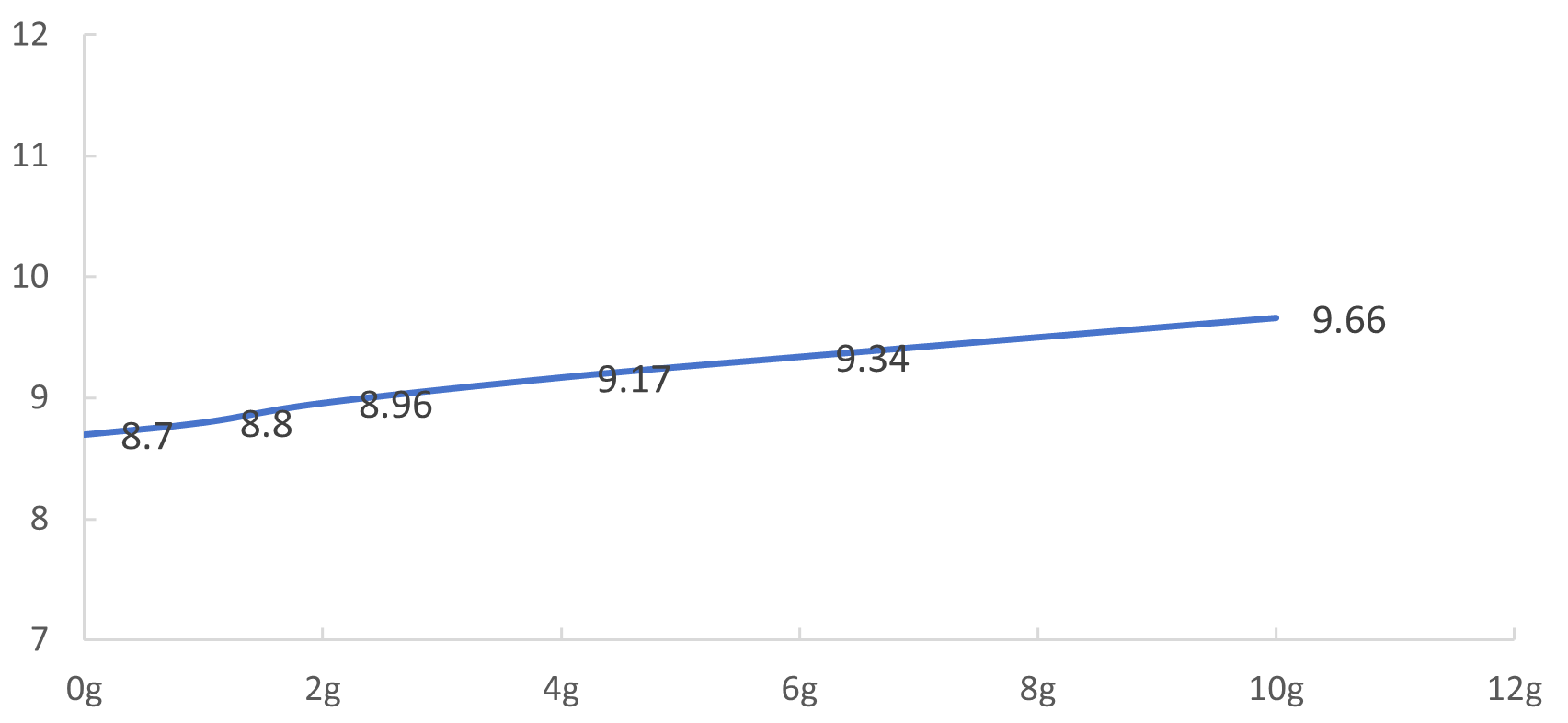
Fig. 1 Alkali content-pH change (25 ℃)
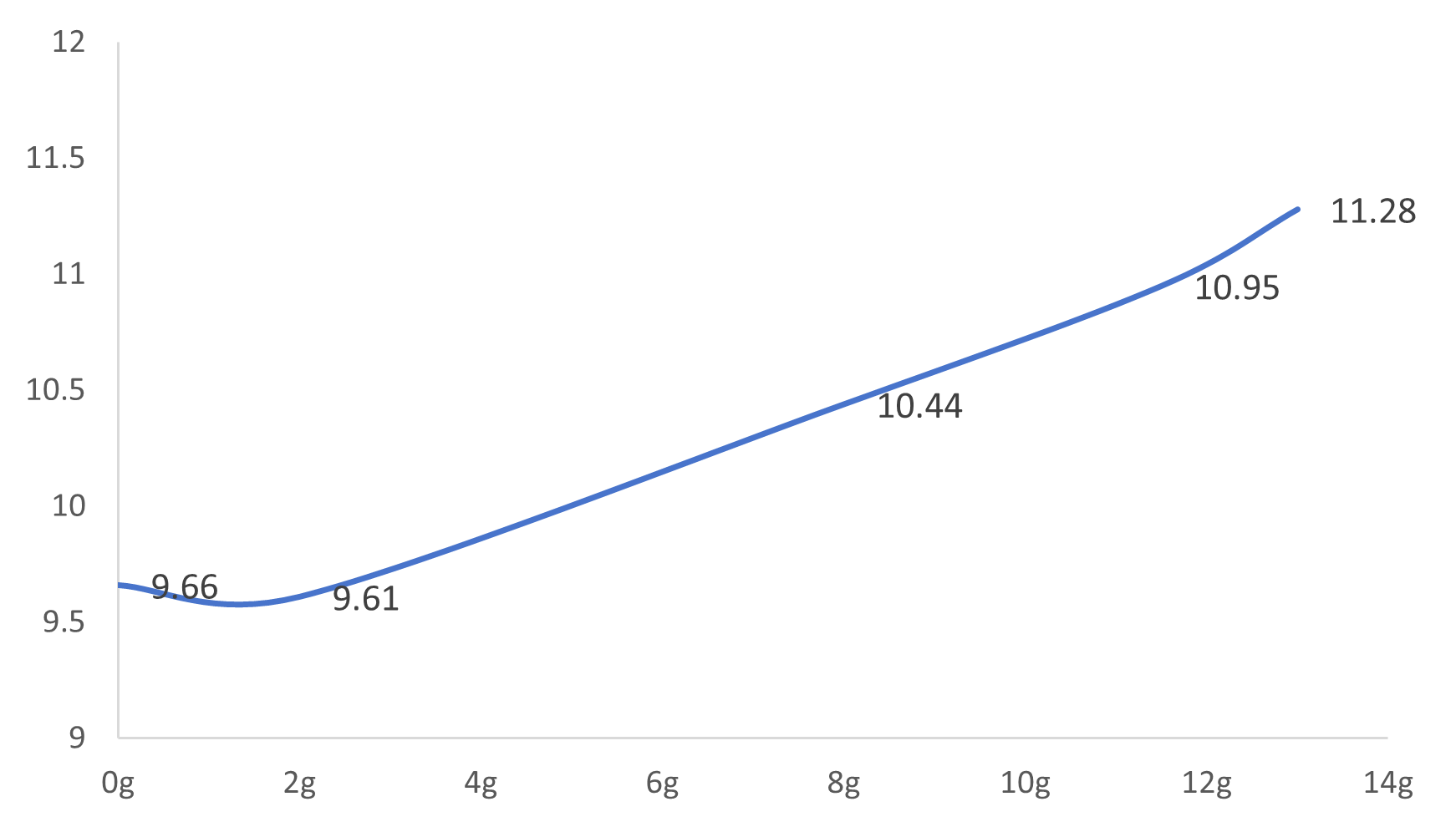
Figure 2 Alkali content-pH change (55 ℃)
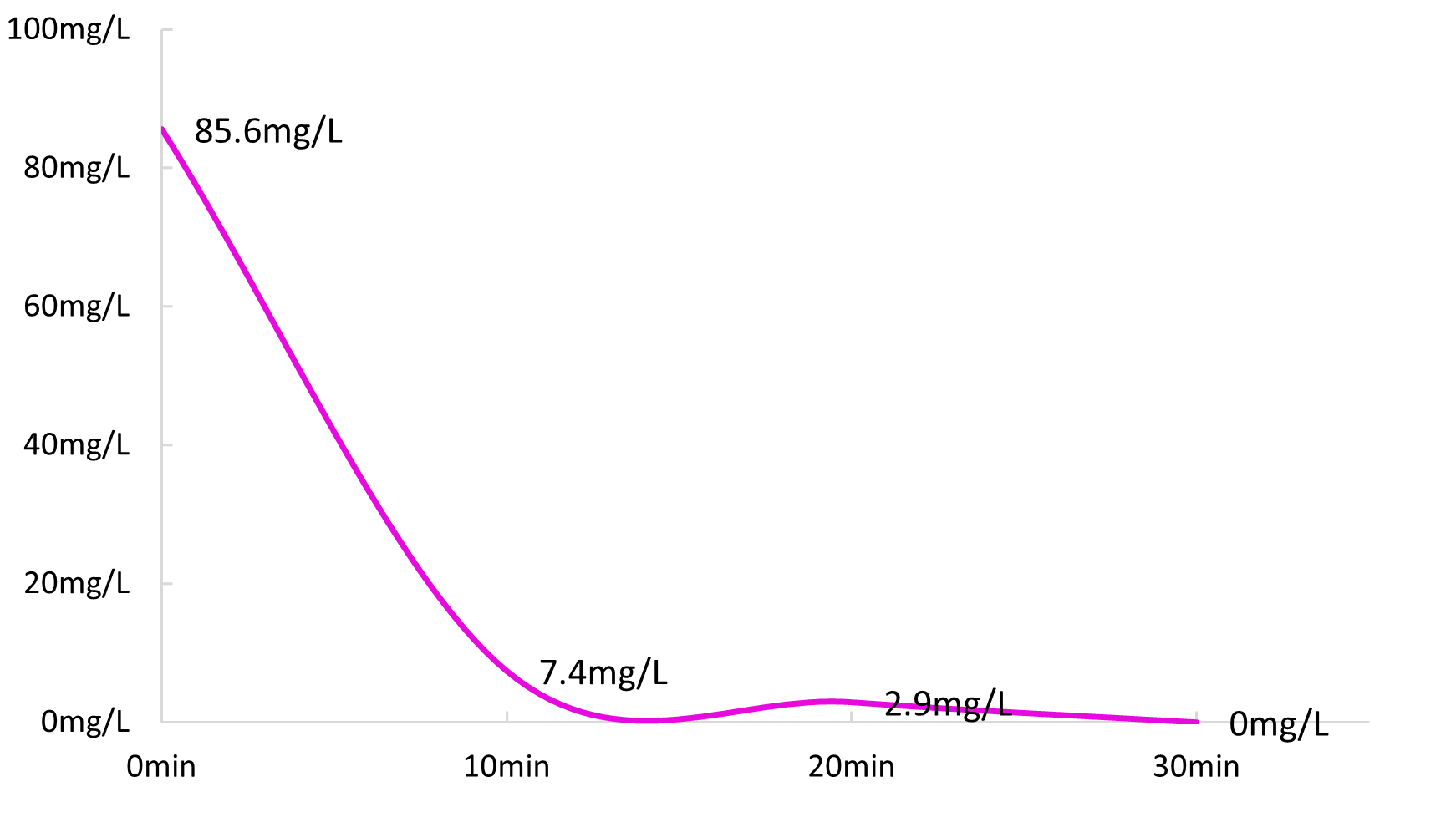
Fig.3 Time-ammonia nitrogen concentration change diagram
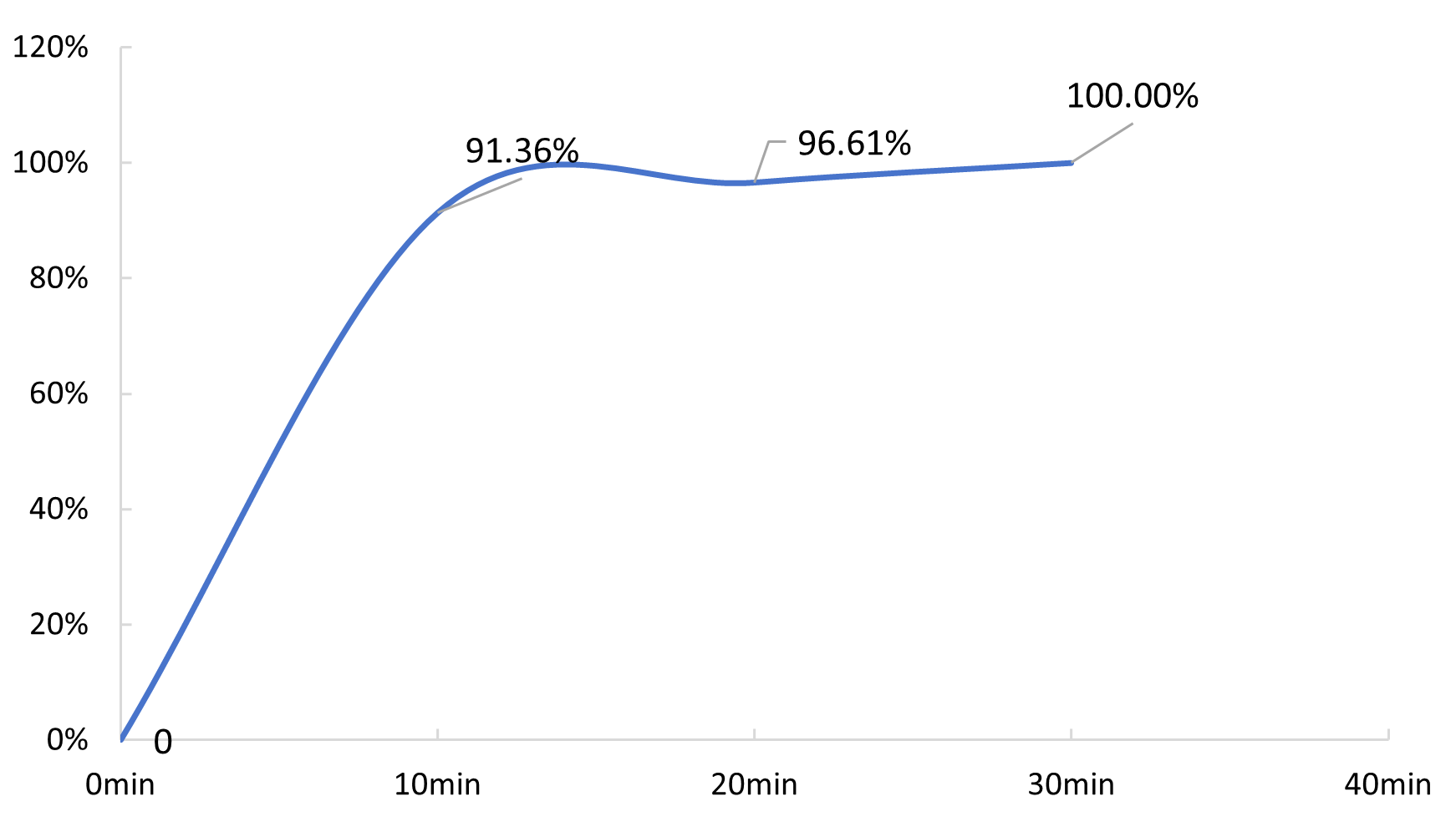
Fig.4 Time-variation of ammonia nitrogen removal rate
6.2 experiment analysis and matters needing attention
(1) adding alkali to adjust the pH of raw water requires adding about 7.5g/L alkali to reach pH = 11.5. the relationship between specific dosage and pH can be seen in fig. 1 and fig. 2. the possible reason is that the components in raw water absorb alkali;
(2) The ammonia nitrogen emission standard of the Project is 10ppm. In this experiment, under the conditions of raw water temperature 70 ℃, pH = 9.29 (alkali addition is about 2g/L) and acid absorption solution pH = 1.5: when the raw water flow rate is 16.8L/h, the primary membrane deamination can meet the drainage requirements within 10 minutes of operation;
(3) During the experiment, it was found that the volume of sulfuric acid absorption liquid gradually decreased, as shown in the following figure. The reason may be that the water temperature increased during the deamination process, while the salt concentration of raw water was high, and the water in the sulfuric acid absorption liquid penetrated into the membrane and discharged with the produced water.
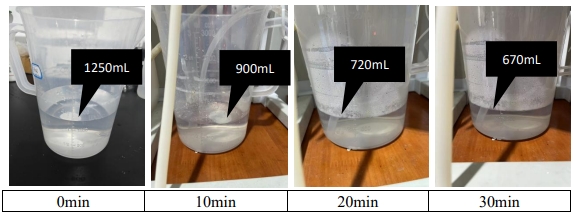
7. Summary and Plan
7.1. Experiment summary
In this small-scale experiment, under the conditions of raw water temperature 70 ℃, alkali addition amount of wastewater about 2g/L(pH = 9.29), and acid absorption solution pH = 1.5: when the raw water flow rate is 16.8L/h, the ammonia nitrogen content of water produced by primary membrane deamination is 7.4mg/L after 10 minutes of stable operation, which meets the ammonia nitrogen requirement of drainage.
7.2. Experimental plan
In this experiment, the membrane deamination requirement is realized under the condition of pH = 9.29 (I .e. the alkali addition is 2g/L). It is planned that the next experiment will explore the best dosage of flake alkali on the premise of meeting the drainage requirement and reduce the drug cost as much as possible.
Latest News












